|
 |
| Encephalitis > Volume 3(3); 2023 > Article |
|
Abstract
In this report, we present a rare case of anti-Ma2-associated encephalitis concurrent with coronavirus disease 2019 (COVID-19) following breast cancer surgery. The patient exhibited minimal clinical symptoms of COVID-19 infection but developed seizures and altered mental status after surgery, leading to diagnosis of a classic paraneoplastic syndrome. This case highlights the possibility of paraneoplastic neurological syndrome even after cancer surgery and the need for careful consideration of post-acute infection syndromes when neurological symptoms occur following an infection.
Paraneoplastic neurological syndromes (PNS) are a result of the immune-mediated effects of remote cancer and are characterized by an autoantibody response against antigens expressed by the tumor [1]. Typically, specific clinical and serological characteristics help identify the type of tumor [1,2]. In particular, anti-Ma2 antibodies, also known as Ma2 or Ta antibodies, are often observed in patients with testicular, ovarian, breast, or lung cancers [3]. They are associated with a specific type of PNS called anti-Ma2–associated encephalitis, which mistakenly attacks the Ma2 protein in neurons and causes paraneoplastic limbic diencephalic and brainstem encephalitis, leading to symptoms such as short-term memory loss, seizures, irritability, cognitive decline, narcolepsy, eye movement abnormalities, and atypical parkinsonism. Some studies have reported that 70% of patients with paraneoplastic encephalitis commonly present with symptoms before their cancer diagnosis, whereas 30% develop encephalitis symptoms after cancer diagnosis [4]. Herein, we present a rare case of a patient positively diagnosed with coronavirus disease 2019 (COVID-19) and anti-Ma2–associated encephalitis after breast cancer resection.
Approval of the ethics committee was exempted by the Institutional Review Board of Ajou University Medical Center (No. AJOUIRB-EX-2023-190) due to the retrospective nature of the study.
A 79-year-old female with hypertension and a history of COVID-19 infection 5 months prior was diagnosed with intraductal papilloma and ductal carcinoma of the right breast 1 month before admission (Figure 1A). The tumor was estrogen receptor 8+, progesterone receptor 8+, human epidermal growth factor receptor-2 2+, KI-67 25%–50% positive, and E-cadherin positive according to immunohistochemistry. The patient underwent a modified radical mastectomy of the right breast and was scheduled to begin chemotherapy 2 weeks after the operation. However, the day before the scheduled chemotherapy session, the patient was found in a stuporous mental state in her house and was brought to our hospital. Upon presentation, the patient had a first onset generalized tonic-clonic seizure with right upper eyeball deviation for 1 minute followed by a gradual regaining of consciousness but remained confused and was in a disoriented mental state. She also exhibited a fever of 38.8°C and positive polymerase chain reaction for COVID-19 on a nasal swab. Her vital signs upon arrival included a blood pressure of 180/91 mmHg, respiratory rate of 21 times/min, and 99% oxygen saturation with 3 L/min of nasal prong oxygenation.
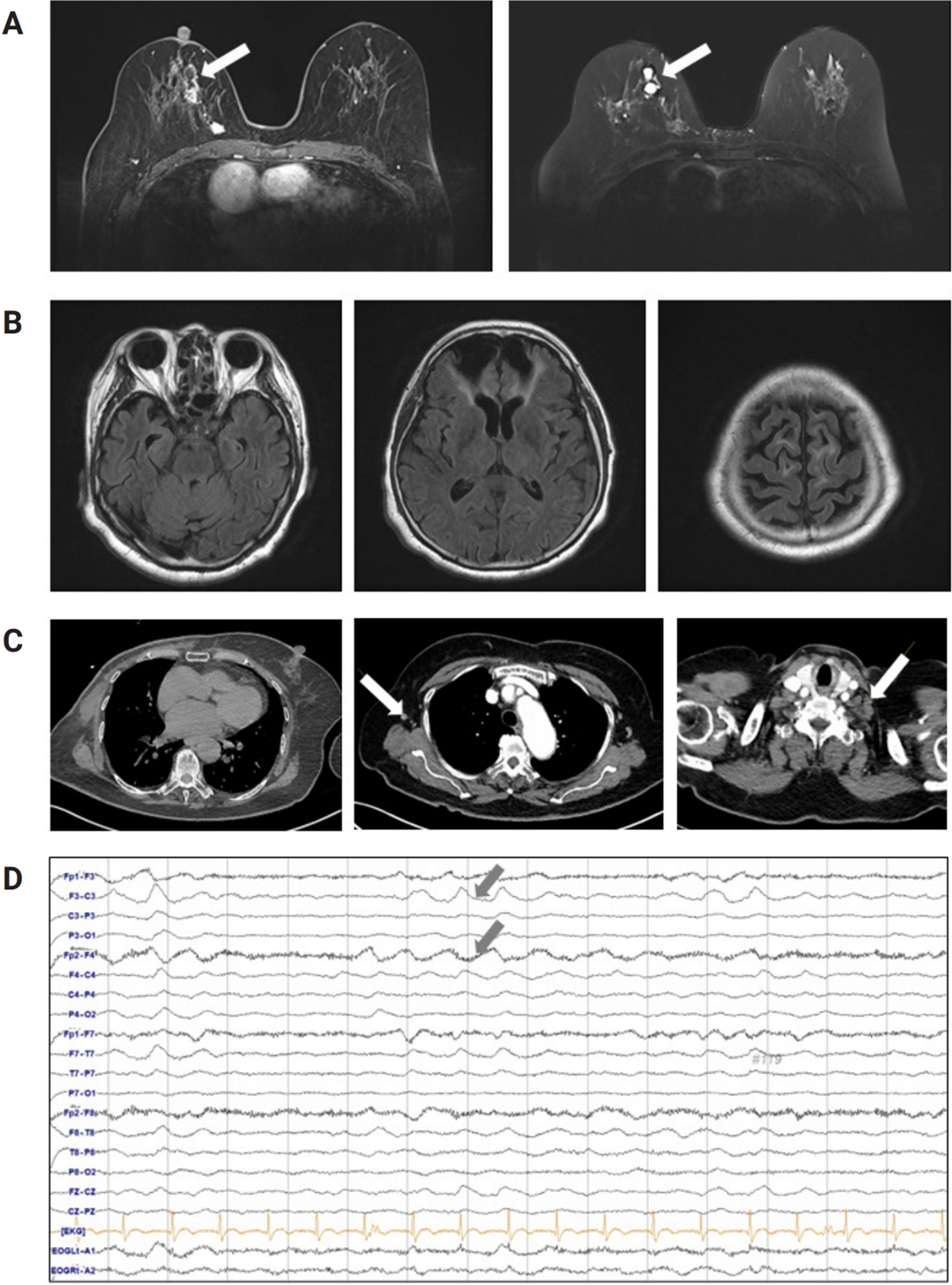
A cerebrospinal fluid (CSF) study revealed neutrophilic pleocytosis (white blood cells, 98 cells/μL; neutrophils, 98%), mild elevation of protein content (55 mg/dL), and normal glucose (55 mg/dL with serum glucose of 123 mg/dL) with a negative polymerase chain reaction test for COVID-19 in the CSF. Serum and CSF screening panels for pathogens such as herpes simplex virus, varicella zoster virus, enterovirus, tuberculosis, Epstein-Barr virus, and syphilis were negative. Brain magnetic resonance imaging showed multifocal encephalomalacia in the bilateral frontal and temporal lobes due to a traumatic head injury 2 years prior but otherwise normal findings (Figure 1B). Chest computed tomography showed postoperative related findings of a right mastectomy. There was no evidence of pneumonia in the bilateral lungs, but there were mild lymphadenopathies of the right axilla and left supraclavicular space suspicious of lymph node metastasis (Figure 1C). For a proper evaluation of the patient’s confused mentality after the seizure, portable electroencephalography monitoring was performed, showing abundant brief runs of moderate-amplitude semirhythmic delta slowing in the bilateral frontal areas (Figure 1D).
For the initial treatment, anti-seizure medications of levetiracetam (1,000 mg/day) and antiviral agents (remdesivir and acyclovir) were administered to prevent seizures and treat suspected viral encephalitis based on the patient’s symptoms and suspected etiology of encephalitis. The follow-up CSF profile 6 days after the first study showed that white blood cell count had decreased to 5 cells/μL, but protein level had increased to 91.9 mg/dL. On the 9th day of hospitalization, subsequent identification of Ma2/PNMA1 antibodies in the patient’s serum suggested an underlying paraneoplastic process, consistent with the patient’s history of breast cancer. Upon diagnosis of PNS, intravenous methylprednisolone pulse therapy at a dosage of 1,000 mg/day was initiated for 5 days, followed by oral prednisolone at 1 mg/kg/day. During the 7-day quarantine period, the patient exhibited impaired cognitive level limited to self-identification. However, after undergoing high-dose intravenous steroid pulse therapy, there was significant improvement in mental status as assessed using the Mini-Mental State Examination score (MMSE), a widely used tool. The initial detailed cognitive function test was performed on the 15th day of hospitalization and revealed a score of MMSE 18 of 30, indicating a significant decline in recall and delayed memory. However, a subsequent exam performed on the 17th day of hospitalization showed a marked improvement in these areas, with the patient scoring 24 of 30. Follow-up electroencephalography revealed occasional brief runs of moderate-amplitude semirhythmic theta/delta slowing. The patient exhibited minimal clinical symptoms of COVID-19 other than fever and showed improvement in encephalitis symptoms by the 24th day of hospitalization. The patient was discharged with an MMSE score of 29 and a modified Rankin scale of 2 and was no longer experiencing cognitive decline or seizures, which were positive outcomes. Five months after discharge, she began receiving chemotherapy for breast cancer in the oncology department.
In this report, we describe an atypical case of a patient who developed anti-Ma2–associated encephalitis concurrent with COVID-19 following breast cancer surgery. Anti-Ma2–associated encephalitis can lead to limbic, brainstem, or diencephalic encephalitis [3]. Considering the patient’s seizures, confusion, and cognitive decline, this appeared to be a classic example of limbic encephalitis attributed to anti-Ma2–associated encephalitis [1,2]. However, our case presents two critical features that warrant future attention: first, the patient was diagnosed with COVID-19 with minimal clinical symptoms other than fever; second, the patient developed PNS after breast cancer surgery. This case provides an opportunity to discuss possible causal relationships between cancer, COVID-19, and paraneoplastic encephalitis.
Numerous autoimmune diseases have been reported in association with COVID-19 infection, including paraneoplastic syndrome or synaptic antibody-related autoimmune encephalitis, similar to our patient’s case of paraneoplastic encephalitis. In one systematic review, 12 N-methyl-ᴅ-aspartate receptor, three glutamic acid decarboxylase-65, two contactin-associated protein-like 2, two myelin, one leucine-rich glioma-inactivated 1, one glial fibrillary acidic protein, and one Yo cases were summarized as causative antigens of COVID-19–related autoimmune encephalitis [5]. The group of medical conditions that occur following acute infection is referred to as post-acute infection syndrome (PAIS) [6]. These syndromes are characterized by symptoms that persist beyond the initial acute phase of the illness and can last for weeks or even months. PAIS can be caused by autoimmune activation, which results from the immune system attempting to target the pathogen, or by bystander autoimmune activation unrelated to the pathogen structures. Autoimmune responses against self-antigens are known to occur after acute infections and may be due to autoreactive T and B cells, which are usually suppressed and become temporarily activated due to impaired regulatory T-cell function, or stimulated by high levels of cytokines in their milieu, disrupting the balance of neuronal and vascular processes [7,8]. Based on our findings, we conclude that our patient developed PNS with T-cell activation after COVID-19 infection, consistent with PAIS.
Most PNS cases emerge after a cancer diagnosis or in patients already diagnosed with cancer [1,2,9]. Interestingly, in our case, the PNS appeared following breast cancer surgery. To the best of our knowledge, this is the first reported case of anti-Ma2–associated encephalitis after resection of breast cancer. A previous report described a 37-year-old woman with anti-Ma2–associated encephalitis at the time of recurrence of cervical squamous cell carcinoma [10]. This is similar to our case in that it was postoperative, but the difference is that the neurological symptoms began when the cancer recurred after a long period of 6 years after surgery. Our patient had a preexisting history of breast cancer and underwent resection, which could have weakened her immune system and made her more susceptible to infections such as COVID-19 [11-13]. One population-based study reported that mortality or morbidity after COVID-19 infection in cancer patients increased significantly in patients diagnosed with cancer less than 1 year prior or in patients who underwent major surgery during the previous 3 months [14].
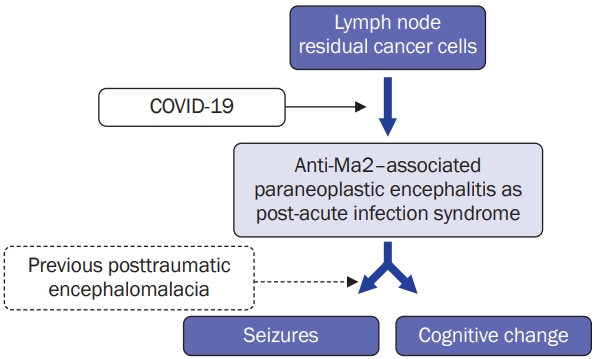
In this case, it can be interpreted that the COVID-19 virus infection stimulated the remaining breast cancer cells in the lymph nodes, triggering an autoimmune response that led to secondary paraneoplastic encephalitis (Figure 2). This created a condition for localized-related seizures in a patient with a history of traumatic brain injury. This suggests that although the exact immune mechanism underlying COVID-19–induced autoimmunity remains controversial, dysregulation of the immune system following COVID-19 infection can serve as a triggering factor for autoimmune diseases. Some studies have shown that mild respiratory infections with COVID-19 can result in patent neuroinflammatory changes, such as white matter selective/enriched microglial reactivity, which can cause multilineage cellular dysregulation in the central nervous system and result in a decline in cognitive function by impairing hippocampal neurogenesis, dysregulation of the oligodendroglial lineage, and myelin loss [15]. In summary, this case highlights the importance of the overall clinical picture and potentially addresses an approach in patients with various medical histories, especially cancer, who present with acute infections with various neurological complications. This underscores the need for a comprehensive diagnostic and treatment approach considering the potential associations and interplay between multiple conditions with a multidisciplinary team including neurologists, oncologists, and infectious disease specialists to ensure the best possible outcomes for patients.
In conclusion, our case report describes a rare occurrence of anti-Ma2–associated encephalitis triggered by COVID-19 immediately following breast cancer resection surgery, which we believe is the first reported case in the world. A takeaway lesson from this case is that a careful approach is necessary when considering the possibility of PAIS when neurological symptoms occur following an infection. Additionally, it should be noted that there is a possibility of PNS even after cancer surgery, and that PAIS can trigger PNS. Therefore, caution must be taken when approaching such cases.
Figure 1.
Representative breast, brain, chest images and electroencephalography of the patient
(A) Breast magnetic resonance imaging (MRI) showing multiple irregularly shaped and heterogeneous margin enhancing masses in the right breast (arrows), suggesting Breast Imaging Reporting and Data System category 6. (B) Brain MRI findings upon presentation. Multifocal encephalomalacia in the bilateral frontal lobe and temporal lobe without any acute infarction or enhancement. (C) Chest computed tomography findings at presentation, with no evidence of pneumonia in the bilateral lungs but showing small lymphadenopathies of the right axilla and left supraclavicular space (arrows). (D) The electroencephalogram findings of the patient. Abundant brief runs of moderate-amplitude theta/delta slowing were noted on the bilateral frontal areas (arrows).
References
1. Graus F, Vogrig A, Muñiz-Castrillo S, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 2021;8:e1014.



2. Binks S, Uy C, Honnorat J, Irani SR. Paraneoplastic neurological syndromes: a practical approach to diagnosis and management. Pract Neurol 2022;22:19–31.


3. Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004;127(Pt 8):1831–1844.


4. Höftberger R, Rosenfeld MR, Dalmau J. Update on neurological paraneoplastic syndromes. Curr Opin Oncol 2015;27:489–495.



5. Samim MM, Dhar D, Goyal S, et al. AI-CoV study: autoimmune encephalitis associated with COVID-19 and its vaccines. A systematic review. J Clin Neurol 2022;18:692–710.




6. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med 2022;28:911–923.



7. Rojas M, Restrepo-Jiménez P, Monsalve DM, et al. Molecular mimicry and autoimmunity. J Autoimmun 2018;95:100–123.


8. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun 2021;4:100100.



9. Fanous I, Dillon P. Paraneoplastic neurological complications of breast cancer. Exp Hematol Oncol 2016;5:29.




10. Ney DE, Messersmith W, Behbakht K. Anti-ma2 paraneoplastic encephalitis in association with recurrent cervical cancer. J Clin Neurol 2014;10:262–266.



11. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer 2021;21:345–359.




13. Lee LY, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21:1309–1316.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0
- 1,599 View
- 35 Download
- ORCID iDs
-
Tae-Joon Kim

https://orcid.org/0000-0001-8451-6634 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print