|
 |
| Encephalitis > Volume 2(2); 2022 > Article |
|
Abstract
Acute disseminated encephalomyelitis is a rare autoimmune demyelinating disease associated with preceding infection or vaccination. Herein, we report a case of refractory fulminant acute disseminated encephalomyelitis that occurred 25 days after Gardasil vaccination (Merck).
Acute disseminated encephalomyelitis (ADEM) is a rare autoimmune demyelinating disease of the central nervous system. ADEM is associated with preceding infection or vaccination [1-3]. Vaccines that can cause ADEM include those for rabies, diphtheria, tetanus, polio, rubella, measles, and mumps [4]. Herein, we report a case of a refractory fulminant ADEM that occurred 25 days after human papillomavirus quadrivalent (Gardasil; Merck, Kenilworth, NJ, USA) vaccination.
A 32-year-old female patient came to our hospital due to urination difficulty and decreased sensation in the lower extremities. A week before her visit, back pain and numbness in the hands and feet occurred but improved after 3 days spontaneously. On the day of the hospital visit, the patient could not urinate at all, and her sensation below the navel was reduced. She had a history of hypothyroidism and had received a human papilloma quadrivalent vaccine 25 days before the visit.
At the time of the hospital visit, initial vital signs included a blood pressure of 153/71 mmHg, heart rate of 99 beats/min, and body temperature of 39.4℃. During the neurologic examination, she was alert and orientated. The cranial nerve examination was unremarkable. She displayed Medical Research Council (MRC) grade IV+ results for all limbs, and she felt paresthesia below the T10 level. The response levels of her deep tendon reflexes were preserved and symmetric in all limbs, and Babinski sign was confirmed bilaterally.
During routine laboratory testing, her white blood cell count was mildly elevated to 10,100/μL, but her erythrocyte sedimentation rate and C-reactive protein level were normal. In addition, kidney, liver, and thyroid function tests were normal. The cerebrospinal fluid (CSF) was clear, with an opening pressure of 140 mmH2O, and showed red blood cells of 5/μL, white blood cells of 95/μL (76% lymphocyte-dominant), total protein concentration of 107 mg/dL, and glucose level of 51 mg/dL (capillary blood glucose level, 107 mg/dL).
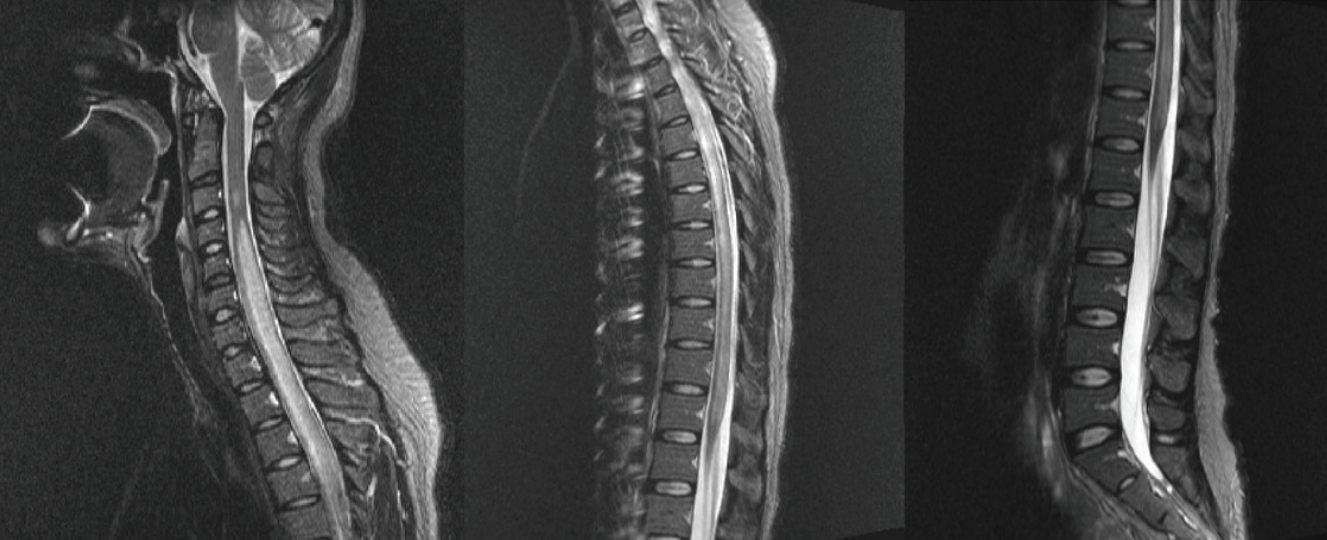
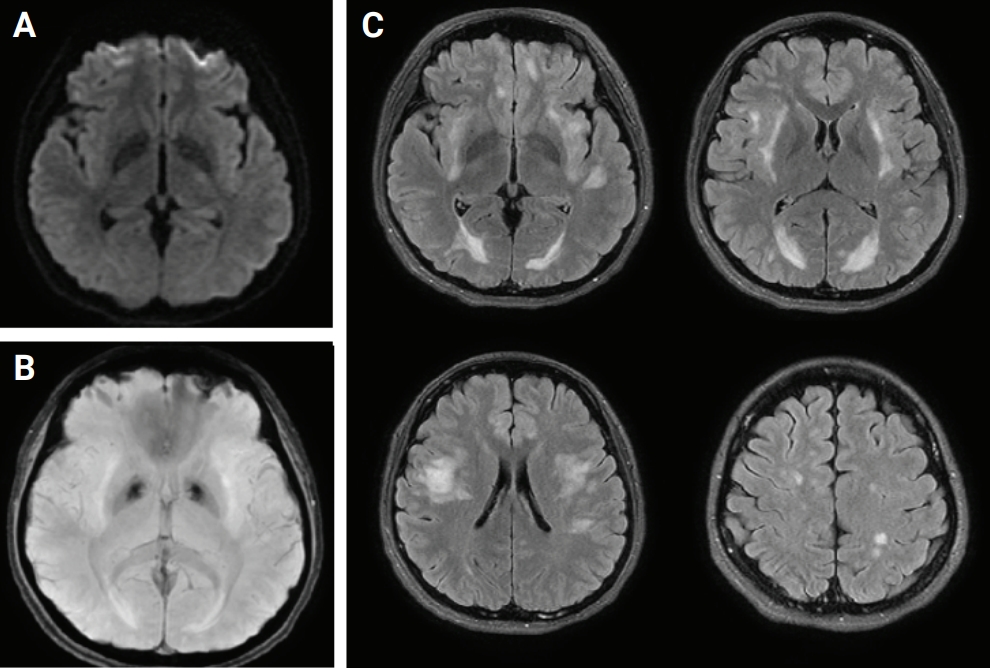
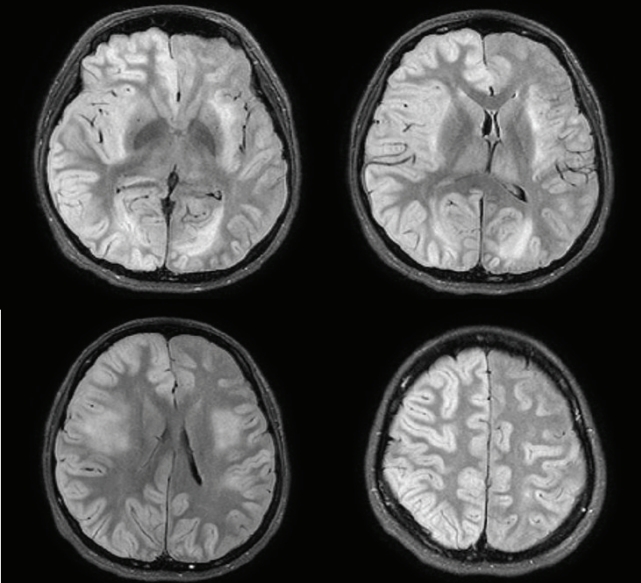
CSF polymerase chain reaction for herpes simplex virus types 1 and 2, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus, and human herpesviruses 6 and 8, and parvovirus B19; CSF gram stain and culture; Bactigen latex agglutination test; India ink stain; CSF tuberculosis/nontuberculous mycobacteria real-time, rapid plasma regain test; fluorescent treponemal antibody absorption test; and specific antibodies to parasites (Clonorchis sinensis, Paragonimus westermani, cysticercus, and sparganum) were all negative. Additionally, oligoclonal bands, aquaporin-4 antibodies, and paraneoplastic antibodies were negative, while her immunoglobulin G index was elevated to 0.85. Spine magnetic resonance imaging (MRI) performed on the day of hospitalization showed diffuse swelling with an extensive T2 high signal change without contrast enhancement (Figure 1), so we initiated intravenous acyclovir, meropenem, and vancomycin therapy as well as steroid pulse treatment for myelitis of an unknown cause. On the 2nd day of hospitalization, the MRC grade was decreased to IV in the upper extremities and II in the lower extremities, respectively. Brain MRI showed diffuse and relatively symmetric T2/fluid-attenuated inversion recovery signal change in the fronto-parieto-occipital periventricular area bilaterally and deep white matter (Figure 2). She experienced convulsions on the 4th day of hospitalization and then showed decreased consciousness. Her systemic blood pressure dropped to 50 mmHg temporarily, but oxygen saturation was normal. Electroencephalography showed generalized continuous theta to delta slowing and no epileptiform discharges, and a follow-up brain MRI taken on the 7th day of hospitalization showed newly developed T2 high signal intensity at the cerebral cortex, thalamus, and cerebellum (Figure 3). We have been treating her using intravenous immune globulin, cyclophosphamide, and rituximab, but she remains in a persistent vegetative state.
ADEM is an autoimmune demyelinating disorder that occurs 2 days to 4 weeks after infection or vaccination as a preceding event [1,3]. Its pathogenesis is the activation of autoimmune responses by molecular mimicry of pathogens and myelin proteins [1,3]. Initially, systemic symptoms, such as fever, headache, and muscle pain appear, and then rapidly progressing neurologic symptoms appear within a few days. Symptoms such as acute onset mental change, motor or sensory deficits, ataxia, and optic neuritis have been reported, and most of these are monophasic [1-3]. Seventy percent of ADEM cases are known to occur following an infection or vaccine, although less than 5% are caused by a vaccine [1,3]. In our patient, there was no history of acute illness or medication history prior to the event, and there was a temporal relationship with vaccine administration [5]. In addition, MRI showed a finding that was suitable for diagnosing ADEM, so we diagnosed this case as an instance of ADEM following Gardasil administration. However, the diagnosis is limited in its applicability because we did not test for myelin oligodendrocyte glycoprotein antibody or ganglioside antibody.
Gardasil is a human papilloma quadrivalent (types 6, 11, 16, and 18) vaccine that prevents cervical, vulvar, and vaginal cancer and genital warts. It was approved by the U.S. Food and Drug Administration in 2006 and is administered as three doses in total [6]. Side effects include local injection site reaction, syncope, headache, nausea, Guillain-Barré syndrome, and venous thromboembolic events, and some cases of ADEM have been reported rarely [7]. Although some patients experience permanent disability or death, ADEM itself has a favorable outcome [1,2]. Like in this case, it is known that the prognosis is poor when there are convulsions or a loss of consciousness [8]. In addition, in cases of ADEM caused by human papillomavirus vaccination reported so far, the prognosis was not bad [9,10]. Wildemann et al. [11] reported that their patient received immunosuppression therapy due to symptom deterioration, but it is very rare to experience mental changes and refractory fulminant ADEM as seen in this case. Therefore, it can be helpful to diagnose and treat patients if we consider that ADEM can occur in patients who receive the Gardasil vaccine.
Figure 1.
Spine magnetic resonance imaging
It shows diffuse swelling with an extensive high signal intensity of the spinal cord.
References
1. Tenembaum S, Chitnis T, Ness J, Hahn JS; International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology 2007;68(16 Suppl 2):S23–S36.

2. Alexander M, Murthy JM. Acute disseminated encephalomyelitis: treatment guidelines. Ann Indian Acad Neurol 2011;14(Suppl 1):S60–S64.

3. Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol 2005;62:1673–1680.


4. Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci 2008;15:1315–1322.



5. World Health Organization (WHO). Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification. 2nd ed. Geneva: WHO; 2018.
6. Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 2006;66:1263–1273.


8. Rodríguez-Porcel F, Hornik A, Rosenblum J, Borys E, Biller J. Refractory fulminant acute disseminated encephalomyelitis (ADEM) in an adult. Front Neurol 2014;5:270.



9. Sekiguchi K, Yasui N, Kowa H, Kanda F, Toda T. Two cases of acute disseminated encephalomyelitis following vaccination against human papilloma virus. Intern Med 2016;55:3181–3184.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0
- 3,728 View
- 33 Download
- ORCID iDs
-
Hakjae Roh

https://orcid.org/0000-0002-0979-4279 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print